Chemical Equations and Stoichiometry
Chemical Equations and Stoichiometry: Overview
This topic covers concepts, such as, Stoichiometry and Stoichiometric Calculations, Calculations Involving Mole-mole Relation, Eudiometry & Calculation of Number of Moles by Eudiometry etc.
Important Questions on Chemical Equations and Stoichiometry
10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and exploded. Amount of water produced in this reaction will be:
The mass of carbon anode consumed (giving only carbon dioxide) in the production of 270 kg of aluminium metal from bauxite by the Hall process is (Atomic mass: ):
In the Haber process of dihydrogen and of dinitrogen were taken for reaction which yielded only of the expected product. What will be the composition of gaseous mixture under the aforesaid condition in the end?
In Haber's process, of dihydrogen and of dinitrogen were taken for the reaction, which yielded only of the expected product. What will be the composition of the gaseous mixture under the aforesaid condition in the end?
Assuming full decomposition, the volume of released at STP on heating (Atomic mass, ) will be:
Liquid benzene burns in oxygen according to the equation
How many litres of at STP are needed to complete the combustion of of liquid benzene?
Liquid benzene burns in oxygen according to the equation,
How many litres of at are needed to complete the combustion of of liquid benzene? (Molecular weight of )
The stoichiometric reaction of of dimethyldichlorosilane with water results in a tetrameric cyclic product in yield. The weight (in ) of obtained is ____________
[Use, molar mass ]
Starting with of , of and of , find the moles of formed().
alloy of reacted with excess of gives of at . What is the of in the alloy?
Atomic weight of
gas is mixed with excess of gas and it is found that is produced. The volume of unused gas is found to be
When x grams of carbon are heated with y grams of oxygen in a closed vessel, no solid residue is left behind. Which of the following statement is correct?
of a gaseous hydrocarbon was exploded with sufficient oxygen. After explosion, there was a contraction of 20mL in volume. On shaking the residual gaseous mixture with , there was a further contraction of 20mL in volume. Calculate the molecular formula. All the volumes were recorded at same temperature and pressure.
If the reaction sequence given below is carried out with moles of acetylene, the amount of the product formed (in ) is
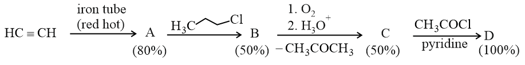
The yields of and are given in parentheses.
[Given: Atomic mass of ]
The treatment of an aqueous solution of of with excess results in a brown solution along with the formation of a precipitate. Passing through this brown solution gives another precipitate . The amount of (in ) is____[Given: Atomic mass of ]
Calculate the millimoles of in solution on the basis of following data:
of solution of was added to solution. The bromine evolved was removed by boiling and excess of was back titrated with of solution of
The reactions are given below.
If of oxygen combine with to form , the mass of in (Atomic mass of ) used in the reaction is
In an experiment, pure carbon monoxide was passed over red-hot copper oxide. so produced, weighed and the weight of copper oxide was reduced by . Calculate the atomic weight of carbon. [ Take molar mass of carbon dioxide = ]
An alloy of aluminium and copper was treated with aqueous . The aluminium dissolved according to the reaction , but the copper remained as a pure metal. A sample of the alloy gave of measured at and pressure. What is the weight percentage of in the alloy?
Give the final answer after rounding off to two significant figures.
When a mixture of and is repeatedly digested with sulphuric acid, all the halogens are expelled and is formed quantitatively. With a particular mixture, it was found that the weight of obtained was precisely the same as the weight of mixture taken. Calculate the ratio of the weights of and in the mixture.
(Note: Report your answer after multiplying with 100 and rounding up to the nearest integer value.)
